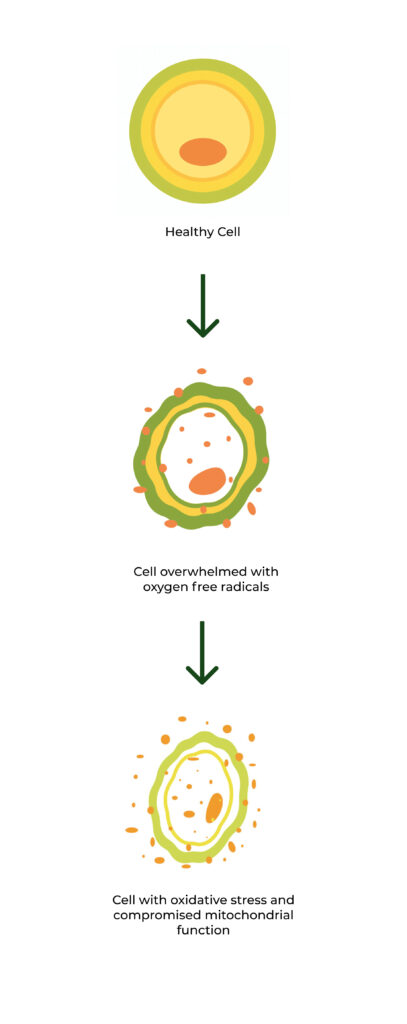
Oxidative Stress, or the presence of excessive oxygen free radicals in the cells, leads to severe cellular damage and compromised mitochondrial health—resulting in the emergence and intensification of chronic disease and symptoms. Eliminating excessive free radicals and restoring balance to the cells is the most effective way to reverse symptoms and regress conditions.
This is the proven science that informs our formulation philosophy.
What the science tells us

Oxidative Stress is caused by excessive production of molecules called oxygen radicals or reactive oxygen species.
These highly reactive molecules are produced in the body during normal metabolism and are extremely dangerous and, if allowed to accumulate, can cause damage to internal organs and lead to chronic disease.

Our body has a natural antioxidant system, but, when overwhelmed, directly leads to oxidative stress.
Oxygen radicals run rampant in our environment, making our bodies vulnerable to increased exposure. Our antioxidant systems also also decline with age, increasing the risk of oxidative stress as we get older.

Cells damaged by oxidative stress have compromised mitochondrial function due to decreased glutathione levels.
Glutathione is our body’s most powerful natural antioxidant. Oxidative Stress diminishes our body’s ability to produce it, leading to compromised Mitochondria (a key factor in symptoms of chronic illness).
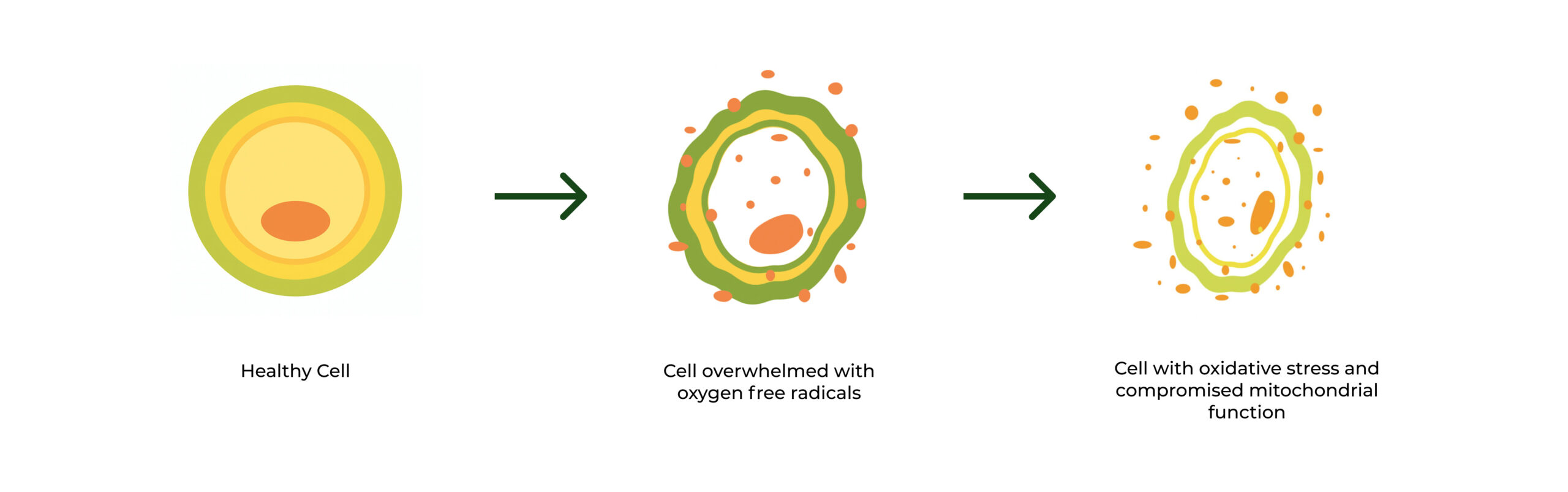
Exposure to free radicals
Its possible to limit exposure to free oxygen radicals, but not to eliminate it. Oxygen radicals are necessary for life, which is why our body has natural enzymatic processes to maintain a healthy balance within cells, including production of natural antioxidants like Glutathione. However, external factors proven to expose the body to more oxygen radicals than the body can handle is what leaves it vulnerable to oxidative damage at the cellular level.
Environmental Factors
Ultraviolet light (UVA) including sunlight
Heavy metals (iron, copper, cadmium, nickel)
Air pollution
Pesticides
Insecticides
Chemicals in everyday plastics
Lifestyle Factors
High sugar foods
Processed foods and meats
Fried foods high in trans fats
Alcohol consumption
Cigarette smoke
Certain medications such as immunosuppressants
There’s also no way to know the level of oxygen radicals the body is exposed to within any given timeframe, given that we aren’t constantly assessing the oxygen radical in the air around us, the products we use, the sunlight we’re basking in, or the pesticides that still linger on the produce we buy at grocery stores.
Reversing oxidative stress with antioxidants
Antioxidants are compounds that break radical chain reactions to neutralize free oxygen radicals and prevent the cellular damage they cause. The body has natural antioxidant systems it relies on, such as the production of glutathione in the cells, but the use of additional compounds (both natural and synthetic) can prevent our natural systems from becoming overwhelmed and replenish diminished sources, but can also maximize those natural processes in order to build greater resilience against oxidative stress.
Antioxidants work by neutralizing harmful oxygen radicals and supporting the body’s own defense systems. But not all antioxidants are created equal. Their effectiveness depends on their ability to enter cells, interact with oxidative compounds like hydrogen peroxide, and repair—not just mask—damage that’s already been done. Traditional antioxidants can still help slow damage caused by oxidative stress—but they often fall short when it comes to reversing it because many antioxidants work indirectly or lose potency before they reach the intracellular environment where damage is occurring.
That’s where R-Dihydro-Lipoic Acid (R-DLA) stands apart. Unlike Alpha Lipoic Acid (ALA), which must be converted by the body before it becomes active, R-DLA is the bioactive form—ready to work immediately, even in environments of extreme oxidative stress.
Clinical research supports this distinction. In one study, R-DLA was shown to reverse lipid peroxidation and preserve mitochondrial function. Another found that R-DLA helped patients achieve full mucosal healing and histologic remission in patients with Ulcerative Colitis—outcomes rarely seen with conventional antioxidant support.
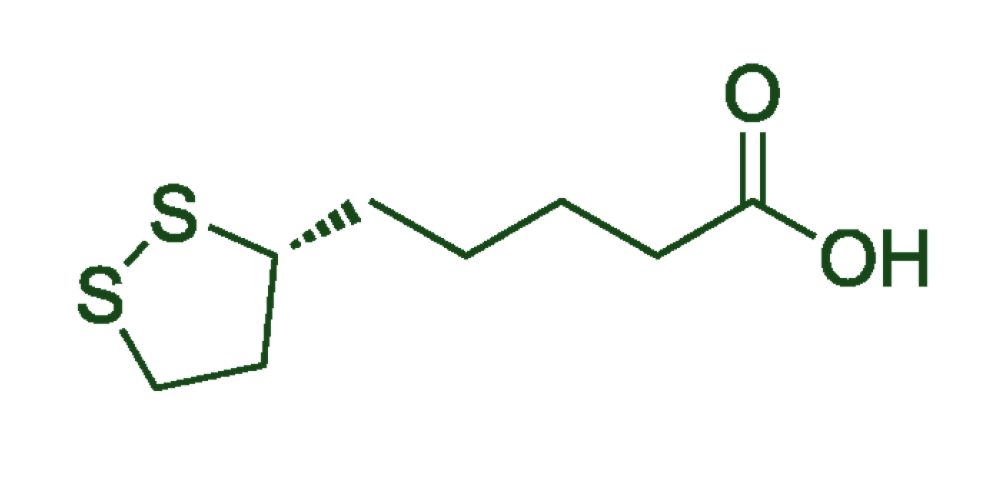
ALA (Alpha-lipoic acid) is the oxidized form of lipoic acid, while R-DLA (R-dihydrolipoic acid) is the naturally-occurring, reduced form of lipoic acid that maintains the molecular structure necessary to both penetrate the membranes of cells and neutralize free oxygen radicals directly.
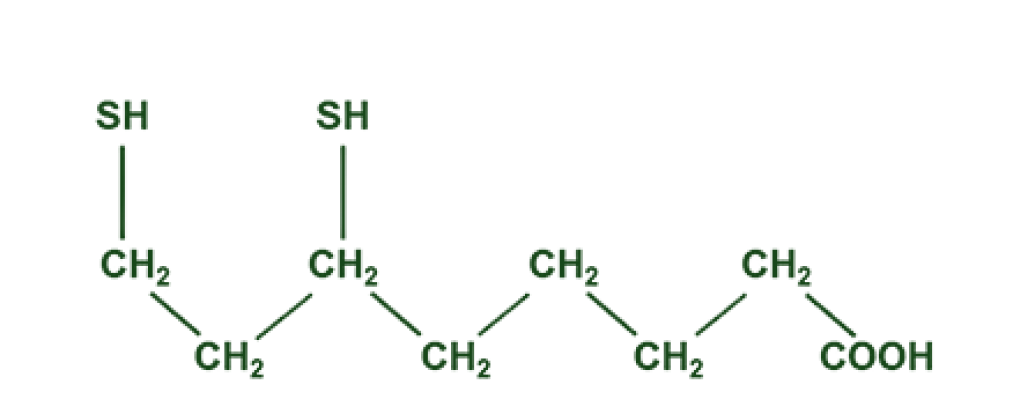
R-DLA
ALA
Research and key studies
Novel Combination Therapy Induced Histological Remission in Patients with Refractory Ulcerative Colitis
Dr. Jay Pravda MD , Michael J Weickert and Lawrence D Wruble
Explores a novel combination therapy for treatment‑resistant ulcerative colitis (UC), targeting histologic remission rather than just symptom control. Thirty‑six patients with moderate-to-severe refractory UC, who had failed conventional treatments (5‑ASA, steroids, immunosuppressants), received nightly combination enemas (mesalamine, budesonide, sodium butyrate, cromolyn sodium) plus daily oral R‑dihydrolipoic acid (R-DLA). The study aimed to assess whether reducing colonic epithelial hydrogen peroxide—a proposed upstream cause of inflammation—could induce true mucosal healing in a relatively short timeframe. A detailed 12‑year follow‑up of one patient demonstrates long-term remission, suggesting potential for durable healing.

High Remission Rates
Histologic remission (complete mucosal healing) was observed in ~85% of the 36 refractory UC patients within an average of 54 days.

Rapid Improvement
Most participants experienced clinical improvements within 3–4 weeks, and continued showing substantial improvement at 8 weeks.

Long-term durability
One detailed patient followed for 12 years maintained normal mucosa and symptom-free bowel function after a single treatment round—with only ongoing low-dose balsalazide.

Novel mechanism
The approach focuses on reducing hydrogen peroxide in colonocytes—thought to be the upstream trigger in UC pathogenesis—rather than suppressing the immune system.
The study concludes that restoring colonic redox balance via this combination therapy may offer a promising alternative to lifelong immunosuppression, with meaningful mucosal healing achieved in most patients and sustained for over a decade in at least one case. For those intrigued by a treatment strategy that goes beyond symptom control and pursues true histological remission, read the full study to explore the therapy details, patient outcomes, and mechanistic insights.
R-Dihydro-Lipoic Acid The Optimal Form of Lipoic Acid
J English, Life Extension
The February 2005 Life Extension report explores the unique benefits of R‑dihydro‑lipoic acid (R‑DLA)—the reduced, biologically active form of alpha‑lipoic acid—highlighting its superior antioxidant and mitochondrial-supportive properties compared to standard alpha‑lipoic acid. The report reviews in vitro and animal studies showing R‑DLA’s ability to directly repair oxidative damage, protect neurons, scavenge reactive oxygen species, and support energy production in heart tissue and blood vessels. It also emphasizes R‑DLA’s potential role in preventing or mitigating age-related illnesses—like diabetes, Alzheimer’s, atherosclerosis, and cardiovascular disease—by improving mitochondrial function, reducing oxidative stress, and enhancing endogenous antioxidant systems.

Enhanced Antioxidant Repair
R‑DLA uniquely repairs oxidative damage—including to alpha‑1 antiprotease—beyond simply neutralizing free radicals.

Superior Radical Scavenging
Compared to alpha‑lipoic acid, R‑DLA more effectively scavenges superoxide and peroxyl radicals in laboratory studies.

Neuroprotective
effects
In cell culture studies exposed to amyloid-beta or iron/hydrogen peroxide, R‑DLA preserved neuronal glucose transport and mitigated oxidative damage—unlike alpha‑lipoic acid alone.

Mitochondrial and cardiovascular support
When combined with agents like vitamin E, R‑DLA significantly improved ATP levels and recovery in heart tissue after oxygen deprivation, supporting mitochondrial and endothelial function.
The report concludes that R‑DLA’s combination of direct oxidative repair, powerful radical scavenging, and mitochondrial protection positions it as a potentially transformative nutrient in addressing age-related diseases like neurodegeneration, diabetes complications, and cardiovascular degeneration. Interested in exploring its therapeutic promise? Read the full article to dive deeper into study designs, molecular insights, and possible applications.
Oxidative Stress and Carbonyl Lesions in Ulcerative Colitis and Colitis‑Associated Colorectal Cancer
Zhiqi Wang, Sai Li, Yu Cao, Zuefei Tian, Rong Zeng, Duan-Fang Liao, Deliang Cao
This comprehensive review synthesizes current insights into how reactive oxygen species (ROS) and secondary electrophilic carbonyl compounds contribute to the development and progression of ulcerative colitis (UC) and colitis-associated colorectal cancer (CAC). It highlights the pathogenic cascade where inflammation-driven ROS cause damage to proteins, DNA, lipids, and activate inflammatory signaling pathways like NF‑κB and p38 MAPK. The paper places particular emphasis on the role of carbonyl byproducts from lipid peroxidation as secondary harmful agents that exacerbate oxidative stress and DNA damage—especially when defense systems like aldo‑keto reductase are compromised. Ultimately, it proposes that this vicious ROS–carbonyl feedback loop accelerates disease progression in UC, contributing to malignant transformation.

ROS trigger inflammatory damage
High levels of reactive oxygen species disrupt DNA, proteins, and lipids—activating pro-inflammatory signaling pathways like NF‑κB and p38 MAPK.

Carbonyls worsen
tissue injury
Lipid peroxidation byproducts (e.g., 4‑HNE, MDA) amplify cellular damage, oxidative stress, and DNA mutations in the colon.

Weakened defense
systems
Reduced activity of detoxifying enzymes (like aldo‑keto reductase) leaves tissues more vulnerable to oxidative and carbonyl stress.

Synergistic disease
models
The study presents a unified model where ROS and carbonyls create a self-reinforcing loop that drives UC progression and cancer risk.
The review concludes that both primary oxidative stress and secondary carbonyl damage critically drive UC’s progression and its transformation into colorectal cancer, underscoring a need for therapeutic strategies that target both ROS and reactive carbonyls. For researchers and clinicians seeking deeper mechanistic insights and novel intervention strategies, delving into the full text will provide valuable models, potential biomarkers, and therapeutic avenues.
Role of Oxidative Stress & Antioxidant Defense in Ulcerative Colitis Patients
SV Rana, S Sharma, K Singh
This clinical study evaluated oxidative stress markers in the red blood cells of 81 ulcerative colitis (UC) patients from North India compared to 85 healthy controls. Researchers measured lipid peroxidation (LPO), antioxidant enzymes (SOD, catalase), and reduced glutathione (GSH). They found that UC patients had significantly elevated LPO, SOD, and catalase, along with notably reduced GSH levels, indicating an imbalance between oxidative stress and antioxidant defense. Importantly, levels did not differ significantly across varying disease activity scores, suggesting consistent oxidative stress regardless of flare intensity.

Lipid peroxidation
significantly increased
UC patients showed elevated LPO markers, highlighting enhanced oxidative damage to cell membranes.

Antioxidant enzymes
were elevated
Levels of superoxide dismutase (SOD) and catalase were significantly higher in UC patients, indicating a compensatory response to increased hydrogren peroxide production.

Glutathione was
depleted
Reduced activity of detoxifying enzymes (like aldo‑keto reductase) leaves tissues more vulnerable to oxidative and carbonyl stress.

Oxidative stress across activity levels
The study presents a unified model where ROS and carbonyls create a self-reinforcing loop that drives UC progression and cancer risk.
The study concludes that ulcerative colitis is associated with a persistent imbalance—elevated oxidative stress and weakened antioxidant defenses—regardless of disease severity. These consistent redox alterations underscore the potential for targeted antioxidant strategies. For those interested in the deeper clinical context, detailed biomarker values, and implications for therapy design, explore the full article for in-depth insights.
The emerging role of oxidative stress in inflammatory bowel disease
Peter Muro, Li Zhang, Shuxuan Li, Zihan Zhao, Tao Jin, Fei Mao, Zhenwei Mao
This review explores how oxidative and nitrosative stress—driven by reactive oxygen (ROS) and nitrogen species (RNS)—play a central role in the development and persistence of inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease. It outlines how immune cells (e.g., neutrophils, macrophages) and damaged intestinal tissues generate excess ROS/RNS, damaging DNA, lipids, and proteins, and disrupting the epithelial barrier via pathways like NF‑κB, MAPK, and Nrf2 . The paper also highlights clinically relevant biomarkers (e.g., malondialdehyde, 8‑OHdG, and serum free thiols) that correlate with disease severity and could aid diagnosis and monitoring. Finally, it reviews antioxidant interventions—such as vitamins C/E, glutathione, and N‑acetylcysteine—suggesting they may help mitigate oxidative damage and inflammation, though more research is needed to establish effective protocols.

Excess ROS/RNS production
Immune activation in IBD leads to overproduction of ROS and RNS, damaging macromolecules and impairing the intestinal lining.

Disrupted signaling pathways
Redox imbalance activates inflammatory cascades, including NF‑κB and MAPK, and compromises cell defenses regulated by Nrf2.

Biomarkers mirror disease severity
Elevated levels of MDA, 8‑OHdG, and reduced serum thiols correlate with inflammation and could be useful in diagnosing and monitoring IBD.

Antioxidant therapy shows promise
Supplementing with antioxidants (like vitamins C/E, glutathione, N-acetylcysteine) has potential to reduce oxidative damage and inflammation—but optimal dosing and clinical protocols are still under study.
The review emphasizes that oxidative stress is a foundational driver of IBD pathogenesis, influencing inflammation, tissue damage, and disease chronicity. While emerging biomarker panels and antioxidant interventions hold promise, robust clinical trials are needed to standardize their use. For healthcare professionals and researchers interested in the antioxidant-treatment potential for IBD, the full article provides valuable in-depth analysis of mechanisms, biomarkers, and therapeutic strategies.

